Throughout World Antimicrobial Awareness Week, we’re featuring key areas of research at Birkbeck relating to the management of diseases. In this blog, we feature the work of former PhD student, Alex Cook, who is looking at new approaches to malaria control.

Alex Cook
Separated by 85 million years of evolution, the parasite Plasmodium falciparum that causes the most deadly form of malaria, is a very different beast to its human host. Yet the challenge for malaria treatments is that they must kill the parasite but not destroy the cells of their human host in which the parasite hides. Malaria is a massive disease burden world-wide. Hundreds of thousands of people are killed each year, the majority of which are children younger than five. In Africa, disruption arising from the COVID-19 pandemic to existing measures also threatens to undo the last decade of malaria control. With resistance to current frontline therapeutics rapidly rising, new drug targets and vaccines are urgently needed.
Malaria-causing parasites are single cells and have a complex life-cycle within both human and mosquito hosts. The many iterations of parasite proliferation that are essential for disease transmission are driven by intracellular machinery called the mitotic spindle, which is built of cytoskeleton components called microtubules. This machinery ensures the correct distribution of replicated chromosomes to the newly produced cells. Targeting of the mitotic spindle by drugs is well-established in a variety of settings – notably human cancers – and components of the malaria proliferative machinery are thus attractive anti-parasite targets.
As part of his PhD work in the research group of Professor Carolyn Moores (Biological Sciences), Alex Cook studied a component of the malaria mitotic spindle machinery, a molecular motor called kinesin-5. Kinesin-5’s are a family of proteins known for their ability to ‘push and pull’ microtubules to create ordered structures within the cell. Alex used a very powerful electron microscope to take images of kinesin-5 molecules – which are around a millionth of a millimetre in size – bound to individual microtubules. He then used computational analysis to combine these pictures and calculate their three-dimensional shape, thereby providing information about how the motors work in the parasite themselves.
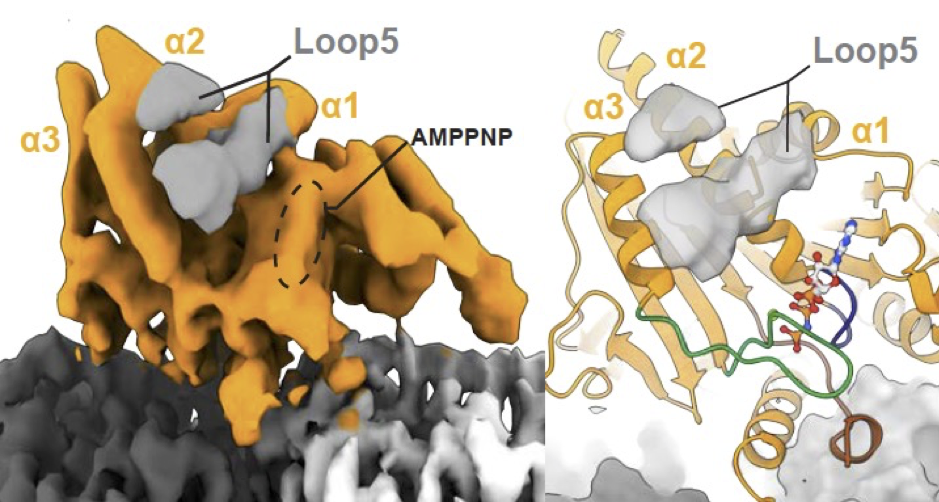
Using this information, Alex – who is co-supervised by Professor Maya Topf and also collaborates with Dr Anthony Roberts, both also in Biological Sciences – showed that although the malaria kinesin-5 motor shares some functional properties with human kinesin-5, there are several key differences that indicate it might be susceptible to specific drug targeting. Confirming this idea, Alex found that a drug-like molecule that blocks human kinesin-5 activity does not affect the parasite motor.
Alex Cook, who is now a postdoctoral researcher at the University of Oxford said: “To uncover new approaches to malaria control, we urgently need to look at new molecules from the parasite. Using high resolution electron microscopy, this first look at a parasite cell division motor will provide a springboard for discovery of small molecules that can disrupt malaria replication.”
Professor Moores commented: “Alex’s hard work, together with vital support from our department’s lab and computational teams, demonstrates the power of electron microscopy to explore medically important challenges.”
Alex’s work was recently published in The Journal of Biological Chemistry (https://doi.org/10.1016/j.jbc.2021.101063). Future directions for the project involve further investigation of specific motor inhibitors, and also of the function of kinesin-5 in the parasite itself, in collaboration with the research group of Professor Rita Tewari at the University of Nottingham.
Further information

Congratulations Alex, Carolyn and Maya.