In May, Birkbeck’s School of Science held ‘Science Saturdays’, a programme of free online talks every Saturday, open to a global audience. In this blog, Tina Wright, Birkbeck PhD student, gives an overview of the talk she attended about pollutants in the air and how they affect the physical wellbeing of humans.
The Science Saturdays talk, ‘Coughing up a lung: pollutants in our air’ delivered by Dr Katherine Thompson, Reader in Biophysical Chemistry, observed how the environment influences physical wellbeing, more specifically, how the atmosphere and its composition of gases and particulate matter, which includes pollutants, interacts with a monolayer of molecules (surfactant) at the air-water interface of the lung to cause respiratory problems.
The content of the talk was a boon to the global cause of tackling climate change and its subsequent effects on worldwide mortality and morbidity rates. It provided information on how to investigate exactly what is happening on a molecular level and systemically in our bodies.
Our atmosphere, pollution and photochemical smog
Our atmosphere predominantly consists of nitrogen (N) and oxygen (O2) along with trace amounts of unnatural and naturally sourced gases, the former being sourced from human processes, for example, car exhaust fumes. These may be from petrol or diesel motors. Petrol motors release some water combined with lots of carbon monoxide (CO), hydrocarbons from incomplete combustion of fuel and nitrogen oxides (NOx) which includes nitric oxide (NO) and nitrogen dioxide (NO2). Diesel motors burn fuel differently, therefore generate a different composition of exhaust gases, such as CO and hydrocarbons, but also release tiny particles that may be dangerous for human health, dependent on particle size.
As I’ve already mentioned, there are some natural sources of trace gases that add to our atmosphere and these include green plants that emit organic pollutants, called volatile organic compounds (VOCs).
Photochemical smog is pollution present on sunny days and has a distinct character. Nitrogen dioxide (NO2) features as a brown/orange gas, visible on the horizon on a clear day. A combination of this with volatile organic compounds (VOCs) and sunlight produces ozone (O3). It is vital for life to have O3 in the stratosphere as it absorbs short wavelengths of light that may damage our DNA, however, when present in the troposphere as a secondary pollutant, the VOC-NOx-O3 relationship keeps its concentration high during warmer months, therefore, adverse effects may occur, notably at the air-water interface of the lung.
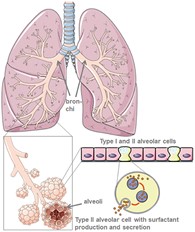
Lung physiology, function and properties on a molecular level
The lung is composed of the trachea, bronchi, bronchioles and finally, millions of alveoli, which are tiny sacs that increase the surface area of the lung for gas-exchange of oxygen (O2) and carbon dioxide (CO2).
Our bodies are wet systems but if the alveoli were lined with pure water, the surface tension (cohesive forces between water molecules), would be too high for expansion of the lung after compression. This issue is resolved by the action of surfactants present as a film on the lung interface that are composed of lipids, proteins, and cholesterol. Its importance is reflected by premature, newborn babies that lack lung surfactant and consequentially, experience difficulties breathing.
What happens when pollutants reach the lung interface?
Dr Katherine Thompson’s research focuses on the interaction of pollutants with the lung. A single layer of molecules (monolayer) of lung surfactant, similar to when it is found on the surface of the lung, may be analysed by the reflection of light. For example, a technique known as Brewster Angle Microscopy, allows the observation of the organization of molecules at the surface or interface, a simile of the lung.
A monolayer is very thin, therefore, the wavelength of light chosen for the reflection experiments should be shorter than the thickness of the monolayer. The wavelength of x-rays (photons) are very short, therefore, it can be used to look at these reflections. Photons may hit a surface and ‘bounce off’ to a detector, comparable to a gymnast jumping and landing on a mat, then coming away. This will give information, in the case of the gymnast, on the thickness of the mat or in the experiment, the single layer of molecules. These experiments may be undertaken whilst the lung surfactant is simultaneously exposed to ozone and a thinning of the monolayer can be observed from rearrangement, breaking and bending of molecules at the surface. Physiologically, this may lead to inflammation of the lung.
This research is of paramount importance in the modern world, where the extent of the damage to our environment, and the subsequent effect on human health, needs further investigation and awareness raised to all.
Further Information